
Company
Technology
Get informed about different departments within the company implantcast and the used technologies.
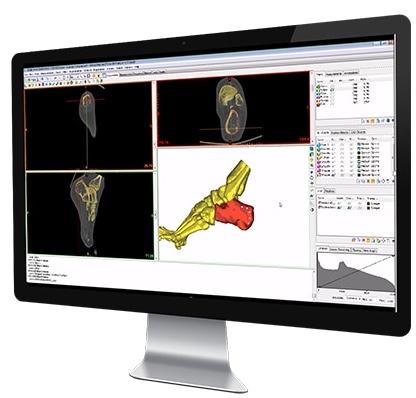
Research and Development
These are the core building blocks of our premium quality products. This, coupled with our capacity for continual improvement is what has made us, and is what keeps us, prepared to meet the ever-changing clinical, legal and regulatory needs of our markets. Close collaborations between implantcast and university hospitals as well as other research organisations all over the world also help us thrive while addressing these challenges.
Our development team, of engineers and technicians drawn from multiple disciplines, is based at our head-quarters in Buxtehude. Using the most up to date technologies we are confident that we can convert a “viable idea” into a finished (registered) product in an efficient and compliant manner.
Standard implant and instrument systems will, of course, always be important to our customers and to us as a company but the need for patient specific endoprostheses is growing rapidly. To ensure cross-fertilisation between the team managing our standard options and the team managing our more individualised offerings, we have integrated these functions within one single research and development department. This way they each get to participate directly in the others work and benefit directly from the others innovations or expertise.



Manufacturing
Precision casting
Each year we cast some 300,000 high-precision robot-based complex components via the lost wax technique which are subsequently tempered prior to x-ray examination. By tearing of samples and metallographic examination of the microstructure we ensure that every implant conforms to all national standards as well as all international standards.
Machining
We have over 100 employees who machine approximately 230,000 high-precision implants from titanium alloy, CoCrMo (cobalt chrome molybdenum) alloy, stainless steel alloy and polyethylene annually. More than 50 ultra-modern metal-cutting machines (some partially automated, others fully automated) are needed to achieve this.
Quality assurance
Using manual gauges as well as fully automated 3D coordinate measuring technology, more than 40 employees check in excess of 820,000 products per year. This important function ensures that each and every implantcast product not only complies with its drawings and other specifications but also with all other standards.
Additive manufacturing
Our more established manufacturing techniques have recently been complemented by bringing additive manufacturing into the mix. It is probably true to say that this technology is among the most innovative to emerge during the 21st century. It enables us to manufacture extremely complex structures and geometries which could not be manufactured by the more established technologies. Show more
Finishing
Only the most highly-trained and specialised employees apply the finish to our devices in order to make them conform to their drawings. Much of the work undertaken in the finishing department is done manually as machinery doesn’t always achieve the best result - which is especially true when it comes to polishing.
At implantcast we always adhere strictly to well-defined specifications and standard operating procedures. To illustrate the point these include everything from new product screening and scoping, new product development, raw material definition and inspection, all stages of manufacture including finished product inspection, logistics including the shipment of goods to customers etc. Thus everything that we do, from start to finish, is guaranteed to take place in a quality-minded environment. Hence we are able to fulfill all national and international demands via our quality management system which is reviewed and, of course, confirmed regularly by our notified body as well as by other relevant international authorities.



