Elbow endoprosthetics
Get informed about all products around the elbow joint.
Intraoperative adjustment of joint tension
ICARA
Radial Head System
Concept
The completely new concept of the ICARA® Radial Head System allows the intraoperative adjustment of the joint tension. With the modular head component, consisting of three parts, the neck length can be adjusted by up to 5mm continuously to restore function and joint stability.
Head components
There are two diameters of head available (19mm and 22mm) which are connected to the stems by a polyethylene snap-locking mechanism.
The centre of rotation of the double inverse prosthesis is located at the level of the radial tuberosity. The prosthesis is self-centering to minimise the potential for malpositioning. The head can swing from neutral to -24° and to +24°.
Stem components
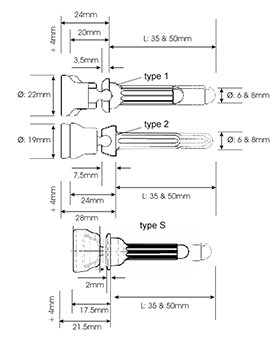
The system includes three different types of stem components with different collar sizes to adapt the system to the different size of bone defect or loss. The type S collar is 2mm thick, type 1 collar is 3.5mm thick and the type 2 collar has a thickness of 7.5mm. The minimal resection for type S is only 17.5mm. The stem components are available in cementless and cemented version each in length 35mm and 50mm and in diameters of 6 and 8mm.
Product details
Materials:
implatan®; TiAl6V4 according to ISO 5832-3 is used for the cementless stem components and head components (the latter being available with TiN-coating)
implavit®; CoCrMo according to ISO 5832-4 is used for the cemented stem components
UHMW-PE according to ISO 5834-2 is used for the PE-inserts in the stem components
Product range:
- 2 head components: 19mm and 22mm in diameter
- 12 cementless stem components: Ø 6mm and 8mm each in type S, type 1, type 2 with two length options (35mm and 50mm)
- 12 cemented stem components: Ø 6mm and 8mm each in type S, type 1, type 2 with two length options (35mm and 50mm)
- Collar thickness: type S: 2mm, type1: 3,5mm, type 2: 7,5mm
non-constrained joint replacement
NES
Elbow System
Design Rationale
The NES (Norway Elbow System) Elbow System is a non-constrained ulno-humeral elbow joint replacement which has been in successful clinical use since 1978. There have been more than 230 implantations in Norway alone since 1994.
The system has been designed to facilitate soft tissue preservation as well as minimal bone resection. This means that the largely intact soft tissue envelope is the main factor influencing stability hence the range of motion is potentially unrestricted.
Components
The cemented humeral implants are available in 4 different sizes and can be freely combined with the 3 cemented ulnar sizes.
The UHMWPE trochlea articulates with a TiAl6V4 axle which is TiN-coated to reduce the polyethylene wear.
Suture holes in the side flanks of the humeral components allow the fixation of bone fragments.
The axle of the preassembled humeral components is secured by a torque wrench.
Product details
Materials:
implavit®; CoCrMo according to ISO 5832-4 (humeral and ulnar components)
implatan®; TiAl6V4 according to ISO 5832-3 with TiN-coating (axle of humeral com ponents)
UHMW-PE according to ISO 5834-2 (PE-spindle of humeral components)
Product range:
- 8 humeral components: left and right, each in sizes small, large, large +5mm and large +10mm
- 6 ulnar components: left and right, each in sizes mini, standard and large
This area is intended for medical professionals only and may only be provided to certain professional personnel.