
Technology
TiN-coating
One layer as wear and allergy protection.
Titanium nitride (TiN) coating
Ideal for all patients regardless of their metal allergy / metal sensitivity status.

solid proven single layer
with more than 300,000 clinically successful implantations worldwide
reliable over a period spanning more than 20 years
no layer failure, no chipping
constant product safety
by validated processes and 100% testing
applied by experienced German coating company
The advantages of this layer:
- excellent biocompatibility
- increased wettability
- extremely high hardness
- can be applied to components intended for cemented and cementless fixation
- high wear resistance
- very good adhesion
- reduced ion release
- minimized polyethylene wear
- chemical long-term stability
The properties at a glance:
- layer type: single ceramic layer
- layer thickness: approximately 5.5µm
- layer colour: gold
- surface hardness: 2400HV
- layer roughness: Ra<0.05µm
- adhesiveness: HF1
Technology
A special arc evaporation technique is used, called physical vapor deposition (PVD) to apply the (ceramic or TiN) layer. The process takes place in a high-vacuum chamber and is computer-controlled for extremely high reproducibility as well as for coating safety.
By adding nitrogen gas into the chamber, the implants are coated. The coating takes place in the vapor phase and is securely anchored within the implant surface to a depth of several atomic layers.
Thus, only the surface of the implant is modified not the material properties of the substrate or its biomechanical functionality.
The ceramic surface coating is wear-reducing, allergy-resistant and biocompatible.
The ceramic TiN coating is applied to the implants by the experienced German coating company DOT GmbH.
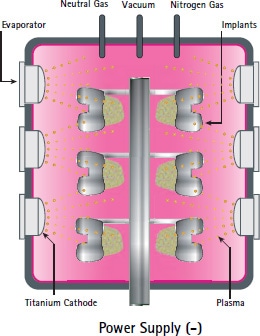
source: Coating dossier DOT GmbH
Reduced wear
The effects of long-term abrasion is one of the main causes of aseptic implant failure and subsequent revision. The TiN-coating reduces abrasion to a minimum. In-vitro wear testing using a knee simulator (according to ISO 14243) demonstrates the very high wear resistance of the hard TiN coating.1
The abrasion of TiN-coated components is only 38% of the wear rate of the uncoated CoCrMo components.2
This difference can only be attributed to the excellent articulating surface quality of the TiN-coating. The high wettability of the coated component further protects the articulation by reducing friction. The ceramic TiN-coating also shows high resistance to scratches in wear tests.
Minimised ion release
Over time all metallic implant components will release ions into their surroundings which can cause allergic reactions in some patients. Nickel (Ni), cobalt (Co) and chromium (Cr) - present in the implant substrate materials - are among the most frequently reported allergy-triggering metals.3 The application of a TiN-coating acts as a barrier to such ion release and minimises the potential for allergic response.4 The ceramic TiN-coating itself is inert and is hence biocompatible.
In-vitro tests of TiN-coated CoCrMo in solution for a defined period of time show that Co, Cr and Ni ion concentrations were below detectable limits.5 The same is not true of uncoated CoCrMo.
In clinical practice, there is no evidence that an allergic reaction was observed when a TiN-coated implant was used with an intact surface.6
This makes the TiN-coating of implant components particularly suitable for patients with sensitisation to nickel, chromium or cobalt.5,6
1 Bader R et al. (2008) Alternative Werkstoffe und Lösungen in der Knieendoprothetik für Patienten mit Metallallergie. Orthpäde 37: 136-142
2 Test report A219/05.1 IMA Dresden. Data on file
3 Eben R et al. (2009) Implantatallergieregister - ein erster Erfahrungsbericht. Orthopäde 38: 557-562
4 Wisbey et al. (1987) Application of PVD TiN coating to Co-Cr-Mo based surgical implants. Biomaterials, 11
5 Prof. Thomas LMU München Final Report Effect of a TiNbN or TiN surface coating on cobaltchromium-molybdenum and stainless steel test specimens regarding the release of nickel, chromium and cobalt: evaluation via eluate analysis and in-vitro cytokine release from peripheral human blood cells, Data on file
6 Baumann A. (2001) Keramische Beschichtungen in der KTEP Standardlösung für Allergiker. JATROS Orthopädie & Rheumatologie 6: 16-17